Número atual: 21(1) - Março 2021
- Imprimir
- Indicar
- Estatísticas
- (0)
Comentários - Como Citar
- Download da Citação
- Artigos Relacionados
-
Outros dos
Autores
Artigo Original
Perfil epidemiológico, clínico e laboratorial de crianças hospitalizadas por doenças respiratórias e análise da evolução após hospitalização
Epidemiological, clinical and laboratory profile of children hospitalized due to respiratory diseases and evolution analysis after hospitalization
Débora Stábile Romero Amais1; Gabriela Dalvi Quintaes Morais1; Andressa Oliveira Peixoto1,2; Fernando Augusto Lima Marson1,3; Naomi Andréia Takesaki1,2; Fernando Belluomini2; Andrea Melo Alexandre Fraga1,2
DOI:10.31365/issn.2595-1769.v21i1p9-15
1. Universidade Estadual de Campinas, Pediatria - Campinas - São Paulo - Brasil
2. Universidade Estadual de Campinas, Unidade de Emergência e Urgência - Campinas - São Paulo - Brasil
3. Universidade São Francisco, Ciências da Saúde - Bragança Paulista - São Paulo - Botsuana
Endereço para correspondência:
fernandolimamarson@hotmail.com
Recebido em: 08/06/2020
Aprovado em: 28/08/2020
Instituição: Universidade Estadual de Campinas, Pediatria - Campinas - São Paulo - Brasil
Resumo
INTRODUÇÃO: Doenças respiratórias são a principal causa de hospitalização e morbimortalidade em crianças sendo que numerosos fatores afetam sua prevalência e gravidade.
OBJETIVO: Verificar o perfil epidemiológico e viral de crianças internadas com doença respiratória e necessidade de oxigenoterapia em uma Unidade de Emergência em Referência Pediátrica.
MÉTODOS: Crianças com idade inferior a 5 anos admitidas no hospital com doença respiratória e necessidade de oxigenoterapia foram incluídos. Foi aplicado ao cuidador um questionário epidemiológico e clínico e, posteriormente, foi realizada a coleta de amostras nasofaríngeas para identificação viral. As análises estatísticas foram realizadas pelos testes Exato de Fisher ou Mann-Whitney usando um nível de significância de 0,05.
RESULTADOS: Vinte e três pacientes foram incluídos sendo que 56,5% eram do sexo masculino, 65,2% tinham um ou mais irmãos =10 anos, 78,7% relataram história de atopia, 39,1% tiveram identificação viral positiva e 60,9% foram hospitalizados. A aglomeração de famílias foi associada ao isolamento viral positivo e menor número de visitas à Unidade de Emergência. Readmissões hospitalares foram associadas ao nascimento prematuro, isolamento viral positivo e história de chiado no peito. O uso de medicação contínua foi frequente entre aqueles que foram hospitalizados, com mais de 1 irmão =10 anos e história de chiado no peito.
CONCLUSÕES: Ser do sexo masculino, ter mais de 1 irmão =10 anos e morar em residência com maior número de indivíduos pode ser fator de risco para doenças respiratórias.
Palavras-chave: Bronquiolite Viral; Vírus Sincicial Respiratório Humano; Influenza Humana; Serviço Hospitalar de Emergência.
Abstract
INTRODUCTION: Respiratory diseases are the main cause of hospitalization, morbidity and mortality in children, and numerous factors affect their prevalence and severity.
OBJECTIVE: To describe the epidemiological and viral profiles of children admitted to hospital with respiratory disease and need for oxygen therapy from a Pediatric Referral Emergency Unit.
METHODS: Children aged less than 5 y.o. admitted to hospital with respiratory disease and need for oxygen therapy were included. An epidemiological and clinical questionnaire was administered for the parent or guardian, followed by the collection of nasopharyngeal specimens for viral identification. Statistical analyses were performed using Mann-Whitney or Fishers exact tests. Alpha was set at 0.05.
RESULTS: Twenty-three children were included being 56.5% were male, 65.2% had one or more (1+) siblings =10 y.o., 78.7% reported a history of atopy, 39.1% had positive viral identification and 60.9% were hospitalized. Household crowding was associated with positive viral isolation and fewer visits to the Pediatric Referral Emergency Unit. Hospital readmissions were associated with preterm birth, positive viral isolation and a history of wheezing. Use of continuous medication was more frequent among those who had been hospitalized, with 1+ siblings =10 y.o. and a history of wheezing.
CONCLUSIONS: To be male, having 1+ siblings =10 y.o. and living in a crowded household could be risk factors for respiratory diseases. Use of continuous medication had a higher prevalence in the presence of history of wheezing and 1+ siblings =10 y.o. The most frequently isolated viruses were respiratory syncytial virus and influenza.
Keywords: Bronchiolitis; Viral. Influenza; Human. Respiratory Syncytial Virus; Human. Emergency Service; Hospital.
INTRODUCTION
Respiratory diseases are the leading cause of hospitalization, morbidity and mortality in children, especially under five years of age1,2. In Latin America, respiratory diseases account for ~80,000 deaths/year: 40% of the cases occur in Brazil3 and most of them are caused by viral bronchiolitis or pneumonia4. Numerous risk factors associated with the presence and severity of respiratory diseases have been described, including: sex, age, low birth weight, breastfeeding, household crowding, passive smoking, family/personal history of respiratory diseases and atopy3. The reason(s) why the disease evolves more severely in some children than in others, even in the absence of a risk factor, is(are) not understood, thus suggesting the complex interaction between host and virus5.
The first studies associating respiratory diseases with etiological agents date back to 19746. Since then, it has been observed that respiratory syncytial virus (RSV), parainfluenza, influenza and adenovirus are the most prevalent viruses in lower respiratory tract infections and the rhinovirus is the main agent of upper airway infections2. Notably, multiple virus infection may occur in acute viral bronchiolitis (AVB)5,7,8.
Several conditions that may be related to the recurrence of respiratory diseases, which leads to new visits to emergency units, hospital readmissions and use of continuous medication9. Therefore, recurrent respiratory diseases have an impact on the healthcare system, patients, caregivers and society. Identifying children who are more prone to develop chronic respiratory diseases may allow early introduction of preventive and therapeutic measures10. Thus, our objective was to verify the epidemiological, clinical and laboratory profile (including viral agent identification) in the Pediatric Referral Emergency Unit (PREU) in children with respiratory diseases and need for oxygen therapy and to associate markers with morbidity in the clinical evolution.
MATERIAL AND METHODS
A transversal study was performed at the PREU in year 2016 and the contact by telephone was done in 2017. The children (≤5 years old) admitted to hospital with respiratory infection and need for oxygen therapy were included. In the study, all patients under the inclusion criteria were included and no exclusion was considered.
A questionnaire was used to collect epidemiological and clinical data, including: self-reported ethnicity; sex; preterm birth; breastfeeding; passive smoking; one or more siblings ≤10 years of age; history of atopy (wheezing and/or dermatitis); history of fever ≥39°C; tachypnea on admission; length of stay in the PREU; destination of the children (home, pediatric ward or intensive care unit - ICU); and attendance at a day-care center. The questionnaire was applied by two medical doctors and authors of the article.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the institution (CAAE:#79533217.9.0000.5404). The informed consent form was signed by a parent or guardian.
Nasopharyngeal specimens were collected by wash or swab methods for viral identification (adenovirus; influenza viruses A and B; parainfluenza viruses type 1, 2 and 3; and RSV) using direct immunofluorescence following the manufacturer's protocol of the Respiratory Virus Screening & ID Kit D3 UltraTM DFA (Werfen MedicalTM, Barueri, São Paulo, Brazil).
One year after hospital discharge, the parents or guardians were called to obtain information on diagnosis of pneumonia after hospitalization, number of visits to the PREU, hospital readmission due to respiratory causes and use of continuous medication (inhaled or topical corticosteroids). If the parents or guardians could not be contacted, the children were included in the cross-sectional analysis of the study.
The statistical analysis included an exploratory study using the mean, standard deviation, minimum, median, maximum, relative and absolute frequency. Association analyses were performed using the Mann-Whitney test and Fisher's exact test. Alpha was set at 0.05.
RESULTS
Twenty-three (17 interviews) children were included, and this group of patients represented the total number of cases. In the sample, the following data was observed according to N (%): 13 (56.5%) males, six (26.1%) with history of preterm birth, 22 (95.7%) with history of exclusive breastfeeding for ≥6 months, three (14.3%) with passive smoking, 15 (65.2%) with one or more siblings ≤10 years of age, 16 (69.6%) with wheezing and two (9.1%) with dermatitis. On admission to the PREU, five (21.7%) and 15 (65.2%) children had a history of fever ≥39°C and tachypnea, respectively. After attending the PREU, nine (39.1%) were discharged from hospital and the others were hospitalized [10 (43.5%) in the pediatric ward and four (17.4%) in the ICU].
Viral identification was positive in nine (39.1%) children, with a single agent isolated in five (55.6%) and ≥2 agents in four (44.4%) children. There were 14 isolates: five (35.7%) RSV, five (35.7%) influenza virus, three (21.4%) parainfluenza virus and one (7.1%) adenovirus.
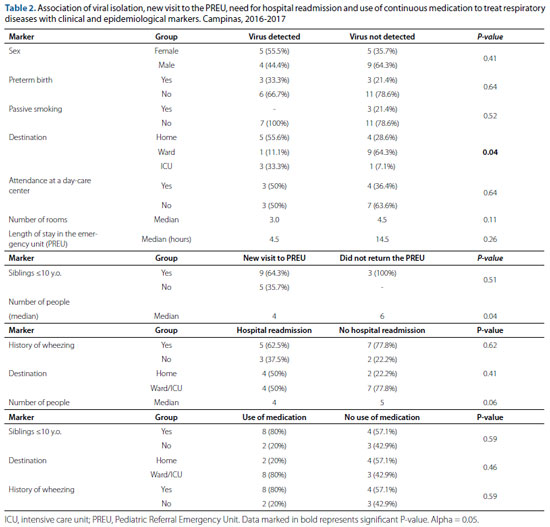
Diagnosis of pneumonia, a new visit to the PREU, hospital readmission due to respiratory causes and the use of continuous medication after hospitalization in the PREU were reported by seven (41.1%), 14 (82.4%), eight (47%) and 10 (58.8%) children, respectively. Age, birth weight, gestational age, number of residents and rooms in the household, respiratory rate, oxygen saturation on admission to the PREU and length of stay at the PREU are summarized in table 1.
Of the nine cases with positive viral identification, five (55.5%) were female, six (66.7%) were non-premature, nine (100%) had no history of passive smoking, five (55.5%) were discharged from hospital, one (11.1%) was admitted to the pediatric ward and three (33.3%) in the ICU (P-value <0.05). Of all cases with positive viral isolation, 50% attended day-care centers. The length of stay at the PREU in the absence of identified virus was
14.5 hours and, in positive cases, it amounted to 4.5 hours (table 2). Of the seven children diagnosed with pneumonia, most (85.7%) had one or more siblings ≤10 years of age. Nine (64.3%) children who returned to the PREU and were readmitted to hospital had one or more siblings ≤10 years of age. The median of the number of residents was six for the children who did not need to return to the PREU and four for those who needed to return to the PREU (table 2).
Regarding preterm children, 60% returned to the PREU and were readmitted to hospital due to respiratory reasons. Of the eight children who were readmitted to hospital, five (62.5%) had a history of wheezing, four (50%) had been discharged at the first visit to the PREU and the others had been admitted to the pediatric ward or ICU (table 2). Positive viral isolation was associated with hospital readmission in four (66.7%) of the six cases. Of the 11 negative cases, only four (36.4%) had been readmitted to hospital.
The use of continuous medication to treat respiratory diseases was used in one (20%) of the premature infants. In addition, of the 10 children on continuous medication, eight (80%) had one or more siblings ≤10 years of age and had a history of wheezing, eight (80%) had been hospitalized (ward or ICU) and two (20%) had been discharged from hospital (table 2). The drug used was inhaled corticosteroids and the time of use was adjusted according to the child's age, between 15 days and four months of medication use. Seventy-five percent of all children who had been admitted to the ICU were on continuous medication; 71.4% of those who had been admitted to the pediatric ward received this therapy; and only 33.3% of those discharged from hospital received this treatment. Age and virus identification of each children are described in table 3.
DISCUSSION
Twenty-three children were included being 56.5% were male, 65.2% had one or more (1+) siblings ≤10 y.o., 78.7% reported a history of atopy, 39.1% had positive viral identification and 60.9% were hospitalized. In short, household crowding was associated with positive viral isolation and fewer visits to the Pediatric Referral Emergency Unit. Hospital readmissions were associated with preterm birth, positive viral isolation and a history of wheezing. Use of continuous medication was more frequent among those who had been hospitalized, with 1+ siblings ≤10 y.o. and a history of wheezing. The clinical diversity in children hospitalized due to respiratory diseases and its evolution after hospitalization corroborate the different clinical profiles, number and prevalence of pathogens, and for therapy response described in the literature11,12,13,14.
Among the patients included in the study, the respiratory diseases were more common in males (56.5%), supporting the literature reviews1,5,15,16;this fact may be explained due to the smaller airway diameter and higher aeroallergen sensitization and IgE level in males17. The history of preterm birth occurred in 26.1% of the cases, while studies show percentages ranging from 13 to 17%2,5. The highest number of preterm can be associated with the selection of a PREU as place to perform the data collection where the higher risk children are attended in the reference region. Curiously, exclusive breastfeeding for six or more months occurred in 95.7% of the cases, which is above the value described in the literature5,18,19,20. Passive smoking occurred in 14.3% of the cases, similar to the findings of Kusel et al.20, showing that passive smoking is associated with disease severity and length of hospitalization21. Most of the children had one or more siblings ≤10 years of age, which is uncommon in literature1. Early contact with other children increases exposure to infectious agents, especially viruses, and can be associated with early wheezing17. Also, most children with pneumonia had one or more siblings ≤10 years of age, which may be justified by a large number of people in the household.
The ICU admission rate was higher (17.4%) than reported in the literature2,5. In a study that analyzed ICU admission due to AVB caused by RSV, the percentage ranged from 6 to 11%5. In contrast, in our study, the rate of hospitalization due to respiratory diseases was lower (43.5%), when compared to hospitalizations due to AVB alone (85.9%)2 - a fact possibly associated with characteristics of the analyzed cases (general care versus children with only AVB). The fever on hospital admission, although the literature evidences an association with the pathogen, occurs in ~40-50% of the cases with lower tract infection, which is higher than the percentage found here2.
Positivity in viral identification was lower (39.1%) than that found in other studies (43 to 86%)1,15,20,22,23. The most frequently isolated viruses were RSV and influenza. The main agent in viral respiratory disease remains controversial in the literature, with some studies reporting RSV2,5,15,23 and other studies citing the rhinovirus1,20,22. A single virus was detected in 55.6% of the positive samples, which is below the values found in the literature (53.9 to 89.5%)1,2,15,22,24. Two or more viruses were detected in 44.4% of the cases, similar to other studies (10.5 to 46.1%)1,2,15,22,24. However, there is no consensus whether disease severity is related to viral identification and/or co-infection with more than one type of virus. Studies have shown that the prevalence of multiviral infection is higher in the group of children who are not hospitalized and do not require oxygen therapy, which may be explained by the fact that bacterial infections may influence viral coinfection5,21,23,24,25,26.
In our data, most cases with positive viral detection were observed in females, although there is a male prevalence for respiratory diseases1,5,15,16. Viruses were more often detected in the non-preterm group, possibly because the number of preterm infants was lower in the sample. Ricart et al. demonstrated that in RSV-positive children, 12.1% were preterm, whereas in RSV-negative children, preterm amounted to 25.4%2. Nevertheless, it should be emphasized that preterm birth is associated with a 7-fold increased risk of AVB caused by RSV and greater severity of infection5,21. Palivizumab prophylaxis may play a key role in the lower frequency of RSV infections in this risk group27. In addition, the cases with detected virus were not exposed to passive smoking contradicting the literature where is reported that passive smoking as a risk factor for lower respiratory tract infection, asthma and AVB3,5,21,26.
Household crowding, measured by an adequate persons-per-room rate, with a satisfactory number of at least four rooms, is a risk factor for acquiring respiratory infections. In developing countries, high birth rate and poor housing conditions justify this fact3,19. Importantly, in our study, a larger number of rooms were associated with lower household crowding, and consequently, with the absence of detected virus.
Kusel et al. report that hospitalization rate in children with negative viral isolation was 29.4%, while in those with positive viral isolation was 70.6%1. Viral detection may determine severity or not, viewing that most cases with positive viral isolation were discharged from hospital, but when hospitalized, children were predominantly treated in the ICU. The length of time in the PREU for the children with no identified virus was longer than in the positive cases, suggesting an association with disease severity and need for treatment at ICUs. In addition, in pharmacological treatment, previous history of wheezing and physical examination on admission with wheezing, probably influences the physician's choice to start bronchodilator or corticosteroid13. A new visit to the PREU due to respiratory reasons in those with one or more siblings ≤10 years of age and attending a day-care center is explained by the increased exposure to respiratory infections and risk of wheezing. However, in the group that did not return to the PREU, all children had one or more siblings ≤10 years of age7,16,17,18,22.
Crowding is a risk factor for viral respiratory diseases3,19,22. However, our data revealed that returns to the emergency unit and readmissions to hospital due to respiratory infections were more frequent in the group with the lowest number of residents at home. A possible explanation to this finding is that a greater number of caregivers would give more assistance to the infant/child and therefore avoid visits to medical services. Hospital readmissions due to respiratory causes were frequent in the preterm group with positive viral isolation. Thus, preterm birth, low birth weight and some types of viral infections (such as coinfection with RSV and rhinovirus) confer a higher risk and severity for viral respiratory diseases, probably associated with a new visit to the PREU and hospital readmission due to respiratory decompensation5,19,21,28.
Nowadays, it is possible to determine the number of virus affecting children by genetics techniques that improved the diagnostic capacity. Also, a high number of virus are described simultaneously in children with respiratory disease and the need of hospitalization29,30. Pediatricians should give special attention for children hospitalized due to respiratory diseases and its evolution during hospitalization mainly in cases where a positive viral infection is present. The disease progression for viral infection can be severe and, in some cases, related with pulmonary sequelae and/or acting as risk factor to other diseases.
AVB is a disease that has controversies regarding its definition and clinical management. In AVB we have the existence of numerous underlying phenotypes with different clinical profiles; therefore, it is essential to know the epidemiological and, if possible, genetic characteristics of the disease, which are correlated with the severity of the disease11,12. The literature describes the possibility of four AVB phenotypes (A, B, C, and D) classified according to clinical characteristics (history of previous wheezing and whether there was a characterization of the first episode, a history of dermatitis), if auscultation pulmonary disease was obstructive, the degree of respiratory distress, length of hospital stay, the number of pathogens identified with greater value in distinguishing the groups between RSV and rhinovirus - main major pathogens involved in the short-term (mainly associated with severity and length of hospital stay) and long-term (mainly associated with the presence of asthma and recurrent wheezing) outcome13. The literature brings us to the reflection that AVB probably represents a set of different diseases that can share biological mechanisms (endotypes) and present themselves with different clinical characteristics (phenotypes). However, there is no consensus in the guidelines regarding the definition of AVB distributed in subgroups that could respond to different therapies and or present different results in the short and long term13,14.
Limitations of the study include: the need of larger sample to achieve a power of at least 80% when Alpha type I error is set at 0.05, technical difficulty in collecting the sample, viral isolation, interruption in the supply of respiratory pathogens viral kits, failure to track clinical progress of some children after discharge and the fact that questionnaires and interviews answered by parents/guardians may have influenced data collection17.
In conclusion, to be a male, having one or more siblings ≤10 years of age and living in crowded households can be risk factors for respiratory diseases. A larger number of residents were associated with a lower number of visits to the PREU. Use of continuous medication was more common in children with a history of wheezing and one or more siblings ≤10 years of age. The most frequently isolated viruses were RSV and influenza.
REFERENCES
1. Kusel MM, de Klerk NH, Holt PG, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J 2006;25:680-6.
2. Ricart S, Marcos MA, Sarda M, et al. Clinical risk factors are more relevant than respiratory viruses in predicting bronchiolitis severity. Pediatr Pulmonol 2012;48:456-63.
3. Prietsch SO, Fischer GB, César JA, et al. Acute lower respiratory illness in under-five children in Rio Grande, Rio Grande do Sul State, Brazil: prevalence and risk factors. Cad Saude Publica 2008;24:1429-38.
4. Hart CA, Cuevas LE. Acute respiratory infections in children. Rev Bras Saúde Matern Infant 2007;7:23-9.
5. Brand HK, de Groot R, Galama JMD, et al. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr Pulmonol 2011;47:393-400.
6. Higgins PB. Viruses associated with acute respiratory infections 1961-71. J Hyg (Lond) 1974;72:425-32.
7. Meissner HC. Viral Bronchiolitis in Children. N Engl J Med 2016;374:62-72.
8. Florin TA, Plint AC, Zorc J. Viral bronchiolitis. Lancet 2017;389:211-24.
9. Fogaça HR, Marson FAL, Toro AA, et al. Epidemiological aspects of and risk factors for wheezing in the first year of life. J Bras Pneumol 2014;406:617-25.
10. Rodríguez-Martínez CE, Sossa-Briceno MP, Castro-Rodriguez JA. Factors predicting persistence of early wheezing through childhood and adolescence: a systematic review of the literature. J Asthma Allergy 2017;10:83-98.
11. Alvarez AE, Marson FA, Bertuzzo CS, Arns CW, Ribeiro JD. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J Pediatr (Rio J) 2013;89:531-43.
12. Alvarez AE, Marson FAL, Bertuzzo CS, et al. Association between single nucleotide polymorphisms in TLR4, TLR2, TLR9, VDR, NOS2 and CCL5 genes with acute viral bronchiolitis. Gene 2018;645:7-17.
13. Dumas O, Mansbach JM, Jartti T, et al. A clustering approach to identify severe bronchiolitis profiles in children. Thorax 2016;71:712-8.
14. Hancock DG, Charles-Britton B, Dixon DL, et al. The heterogeneity of viral bronchiolitis: A lack of universal consensus definitions. Pediatr Pulmonol. 2017;52(9):1234-40.
15. Mansbach JM, McAdam AJ, Clark S, et al. Prospective multicenter study of the viral etiology of bronchiolitis in the Emergency Departament. Acad Emerg Med 2008;15:111-8.
16. Bonfim CM, Nogueira ML, Simas PVM, et al. Frequent respiratory pathogens of respiratory tract infections in children attending daycare centers. J Pediatr 2011;87:439-44.
17. Dela Bianca A, Wandalsen G, Mallol J, et al. Risk factors for wheezing disorders in infants in the first year of life living in São Paulo, Brazil. J Trop Pediatr 2012;58:501-4.
18. Saini A, Kaushik JS, Arya V, et al. Predictors for critical care admission among children presenting to emergency department with de recurrent wheezing. J Emerg Trauma Shock 2017;10:26-30.
19. Martins AL, Nascimento Dda S, Schneider IJ, et al. Incidence of community-acquired infections of lower airways among infants. Rev Paul Pediatr 2016;34:204-9.
20. Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infection, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol 2007;119:1105-10.
21. Alfonso EA, Marson FAL, Bertuzzo CS, et al. Epidemiological and genetic characteristics associated with the severity of acute viral bronchiolitis by respiratory syncytial virus. J Pediatr 2013;89:531-43.
22. Souza LS, Ramos EAG, Carvalho FM, et al. Viral respiratory infections in young children attending day care in urban Northeast Brazil. Pediatr Pulmonol. 2003;35:184-91.
23. Sly PD, Jones CM. Viral co-detection in infants hospitalized with respiratory disease: is it important to detect? J Pediatr 2011;87:277-80.
24. Martin ET, Kuypers J, Wald A, et al. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respir Viruses 2011;6:71-7.
25. Martin ET, Fairchok MP, Stednick ZJ, et al. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis 2013;207:982-9.
26. Chorazy ML, Lebeck MG, McCarthy TA, et al. Polymicrobial acute respiratory infections in a hospital-based pediatric population. Pediatr Infect Dis 2013;32:460-6.
27. Narbona-Lopez E, Uberos J, Checa-Ros A, Rodriguez-Belmonte R, Muñoz-Hoyos A. Prevention of syncytial respiratory virus infection with palivizumab: descriptive and comparative analysis after 12 years of use. Minerva Pediatr. 2018;70(6):513-518.
28. Hasegawa K, Mansbach JM, Teach SJ, et al. Multicenter study of viral etiology and relapse in hospitalized children with bronchiolitis. Pediatr Infect Dis J 2014;33:809-13.
29. Tsou P, Vadivelan A, Kovvuri M, et al. Association between multiple respiratory viral infections and pediatric intensive care unit admission among infants with bronchiolitis. Arch Pediatr. 2020;27(1):39-44.
30. Hancock DG, Charles-Britton B, Dixon DL, et al. The heterogeneity of viral bronchiolitis: A lack of universal consensus definitions. Pediatr Pulmonol. 2017;52(9):1234-40.